Einführung
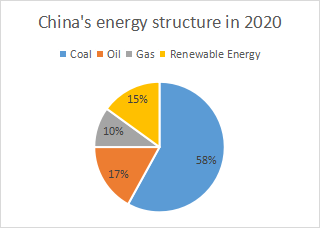
Unter fossiler Energie versteht man Erdöl, Erdgas und Kohle. Aufgrund der Ressourceneigenschaften Chinas, reich an Kohle und arm an Öl und Gas zu sein, ist Chinas fossile Energie stark auf Kohle ausgerichtet. Der umfangreiche Einsatz von Kohle wird zahlreiche Auswirkungen auf die Umwelt haben, insbesondere bei der Verbrennung, bei der viele Luftschadstoffe freigesetzt werden, darunter Feinstaub, Kohlendioxid, Schwefeldioxid und Stickoxide, was zu Umweltverschmutzung führt.
Unter anderem können Stickoxide (NOx) als Hauptschadstoff in der Atmosphäre sauren Regen, photochemischen Smog, städtischen Dunst, Abbau der Ozonschicht und viele andere Umweltprobleme verursachen. Es kann sich leicht mit Hämoglobin im menschlichen Körper verbinden und so den Sauerstofftransport im Blut blockieren, was zu einer Lähmung des Zentralnervensystems führt und die Herz-Kreislauf- und Lungenfunktionen des Menschen gefährdet.
Zu den in der industriellen Produktion weit verbreiteten NOx-Kontrolltechnologien gehören die stickstoffarme Verbrennungstechnologie, die selektive nichtkatalytische Reduktionstechnologie (SNCR) und die selektive katalytische Reduktionstechnologie (SCR).
2. Einführung der CO-SCR-Technologie
CO-SCR technology reduces NOx to N2 by using carbon monoxide (CO) as a reducing agent. CO is a reducing gas that is widely present in sintering and coking flue gas and vehicle exhaust. It is also a colorless and odorless toxic gas, which can cause poisoning when the CO concentration in the air exceeds 0.1%. Using CO instead of NH3 for selective catalytic reduction of NOx can not only reduce pollution control costs but also eliminate NO and CO in the flue gas, achieving waste treatment through waste.
2.1 CO-SCR technology principle
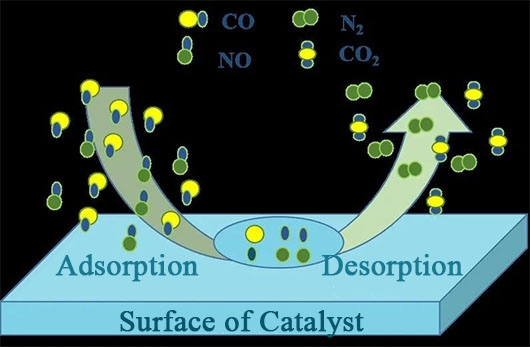
The reaction process of CO reducing NO can be divided into four steps: adsorption of reactant molecules (CO and NO first undergo gas-phase diffusion and contact with the catalyst surface, and are adsorbed by the unsaturated metal active sites on the catalyst surface, forming NO(a) and CO(a) species, while CO and NO gradually diffuse into the pore structure of the catalyst as the reaction continues); dissociation of adsorbed molecules (when the reaction reaches a certain temperature, active NO(a) is decomposed into N(a) and O(a) species); recombination of surface active substances and desorption of product molecules (CO(a) is oxidized by the active O(a) species to generate CO2, while the active N(a) species combines to generate N2, and the final products CO2 and N2 generated by the reaction are discharged from the flue). Meanwhile, the combination of other active species can produce by-products such as N2O and O2, and the specific steps are as follows:
Adsorption of reactant molecules:
CO(g) → CO(a)
NO(g) → NO(a)
Dissociation of adsorbed molecules:
NO(a) → N(a) + O(a)
Recombination of surface active substances and desorption of product molecules:
CO(a) + O(a) → CO(g)
N(a) + N(a) → N2(g)
N(a) + NO(a) → N₁O(a)
N:O(a) → NO(g)
N.O(a) → N₂(g) + O(a)
2.2 CO-SCR catalyst
The catalyst is the key material in the entire catalytic reaction system. Currently, in the CO-SCR denitration technology that uses CO as a reducing agent to remove NOx, commonly used catalysts include noble metal catalysts, single transition metal catalysts, and composite transition metal catalysts. Noble metal catalysts generally refer to platinum, palladium, rhodium, iridium, silver, etc. Noble metals are often present in a nanometer state as catalysts, or they can be supported on carriers or exist on molecular sieves by ion exchange. The oxides of non-noble metals such as copper, cobalt, iron, chromium, and manganese have good activity for removing nitrogen oxides. Single metal catalysts often have the disadvantages of a narrow reaction temperature window, poor reaction activity under oxygen-rich conditions, and poor resistance to SO2 poisoning. Composite metal catalysts doping appropriate additives can improve these problems to some extent. Compared with single transition metal oxide catalysts, bicomponent or multicomponent composite transition metal oxide catalysts often have higher dispersion of active components and better activity and stability of the catalysts.
2.3 CO-SCR technology characteristics
The CO-SCR technology uses the harmful gas CO in flue gas to reduce nitrogen oxides and achieve flue gas denitrification. Its advantages mainly include:
(1) CO, as a reducing gas, has strong reducibility at 100-400℃ and is a good NOx reducing agent;
(2) the reducing agent comes from the flue gas itself, and the denitrification cost is greatly reduced;
(3) it also reduces the emissions of CO and NOx, achieving the goal of "treating waste with waste";
(4) there is no ammonia escape, and no secondary pollution is caused.